Who is NIPT for?
Eurofins Biomnis follows the international recommendations of the American Congress of Obstetricians and Gynecologists (ACOG) and the Society for Maternal–Fetal Medicine (SMFM) with regard to the non-invasive prenatal testing of high-risk patients.
When can Ninalia NIPT be conducted?
Ninalia NIPT can be carried out at any point in the pregnancy from Week 10 (gestational age).
What are the performance data for Ninalia NIPT?
Since October 2014, Eurofins Biomnis has chosen to collaborate with the company Illumina, a world leader in DNA sequencing, for non-invasive screening for the main chromosomal abnormalities.
The new VeriSeq NIPT method offers the best performance for non-invasive screening for the main chromosomal abnormalities.
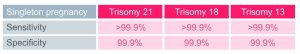
Data from the supplier Illumina, July 2019
How to prescribe Ninalia NIPT?
The following documents are needed to conduct Ninalia NIPT:
- Medical prescription and justification as to why the test is indicated
- The specific Biomnis information form for Ninalia NIPT, properly completed with the required clinical information. This form also contains the specific consent form that needs to be co-signed by the patient and the prescribing physician
- Report from the 1st trimester ultrasound
How is the sample collected?
This sample requires 1 specific “Streck” tube.
Sample type: Whole blood (1 x 10 mL).
How are the results returned?
The results are reported directly to the physician who prescribed the test within a period of five working days (Monday to Friday) from sample receipt at Eurofins Biomnis.
Who can I contact if there are questions about the results?
You can contact Eurofins Biomnis at international@biomnis.eurofinseu.com
Is there a risk of false positive and false negative results?
Rare cases of false positives and false negatives have been described in the following contexts:
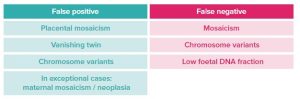
Ninalia NIPT currently offers the best performance for non-invasive screening for the main chromosomal abnormalities.
I have not found the answer to my question: who can I contact?
The laboratory specialists approved for prenatal diagnostics and responsible for Ninalia NIPT at Eurofins Biomnis are available to work with you to ensure the best possible implementation of this test, from assessment of the relevance of the test in the context of the patient’s medical records, to reporting the results
You can contact Eurofins Biomnis at international@biomnis.eurofinseu.com
Eurofins Biomnis: who are we?
European leader in the sector of specialised medical pathology, Eurofins Biomnis carries out over 39,000 analyses per day from a range of over 3,000 available tests, including specialised tests for which the company has the appropriate authorisations.
Founded in 1897 by Marcel Mérieux, Eurofins Biomnis remains the leader in the field of specialised medical pathology in France. It has kept its position through continuous technological innovation and investment, particularly in areas such as female biology, oncology and personalised medicine, as well as chromosomal and molecular genetics.
With 120 years of expertise and innovation in the service of medical pathology, Eurofins Biomnis is the core of the Eurofins Clinical Diagnostics division in Europe and has plans for international development.
Screening for T21 - 15.11.2023


